Menu

After an intense selection process at the European Innovation Council NecstGen, IBSAL and Trince were selected with their Penphomet project, Penphomet is one of the 27 that has been awarded. Led by Trince, the consortium aims to revolutionize cell therapy manufacturing by integrating nanotechnology, optics, and microfluidics.

Cancer immunotherapy has emerged as a promising approach to treat cancers by harnessing the patient’s own immune system. Adoptive T cell therapy, in which patient-derived T cells are genetically engineered with a chimeric antigen receptor (CAR), is particularly attractive. However, solid tumors present additional challenges such as tumor antigen heterogeneity, limited infiltration, and immunosuppression. Mesenchymal stromal cells (MSCs) can home to tumors and deliver anticancer agents, such as cytokines, to enhance the efficacy of CAR T cells.
Ex vivo genetic modification of T cells and MSCs has traditionally been performed with viral vectors, but these have challenges such as safety concerns, high costs, production challenges, and limited flexibility. Trince is developing PEN photoporation as a gentler intracellular delivery technology, resulting in improved transfection yield, better phenotypic profile, faster proliferation, and better killing potential in CAR T cells. Fast proliferating high-quality CAR T cells are expected to reduce the manufacturing time and costs, and offer faster treatment to more patients. In this project, the PEN photoporation technology will be brought to TLR6 by developing hard- and software for automated high-throughput transfections of T cells and MSCs. The technology developped by Trince will be extensively tested and validated in the cGMP compliant laboratories of NectsGen and Ibsal for the genetic engineering of T cells and MSCs. The Penphomet project aims to further develop the photoporation based transfection platform for more cost-effective and potent cell therapies.
Trince is developing phothermal electrospun nanofiber (PEN) photoporation as a gentler intracellular delivery technology. In this approach, cells are cultured on electrospun polycaprolactone (PCL) nanofibers containing photothermal iron-oxide nanoparticles (IONPs). Upon adding the payload molecules and applying pulsed laser irradiation, the IONPs generate photothermal effects which permeabilize the cells, allowing entry of the payload molecules in the cells. The transfected cells are finally collected from the fibers for further testing and therapeutic use.
The technological objectives in this project are aimed at bringing PEN photoporation from TRL4 to TRL6, while the Business objectives are aimed at preparing commercialization and market deployment. By the end of the project a fully automated and validated high-throughput prototype system will be available for installation at a centralized cell production facility or ready for integration in point-of-care cell manufacturing equipment.

NecstGen is a non-profit CDMO and centre of excellence for Cell and Gene Therapy, located in a purpose-built GMP facility on the largest bio-cluster in the Netherlands, Leiden Bio Science Park. NecstGen provides critical contract development, manufacturing and rental services to academic and industrial therapy developers to deliver a new generation of therapies to patients.
NecstGen offers:

The Institute of Biomedical Research of Salamanca (IBSAL) is part of IECSCYL (The Fundación Instituto de Estudios de Ciencias de la Salud de Castilla y León) and is one of the Biomedical Research Institutes accredited by the Carlos III Institute from the Spanish Ministry of Health (Order of February 17, 2014). IBSAL’s mission is to develop clinical and translational research, promoting synergy between clinical and basic research groups and optimizing resources through shared services and efficient management structures. One of the 6 areas of the Institute is Gene, Cell and Transplant Therapy, coordinated by Prof. Sánchez-Guijo. The location where the project tasks will be carried out is the Hematology Department of the University Hospital of Salamanca (HUS), also chaired by Prof Sanchez-Guijo. The Department provides services a.o. in cell therapy, includes a GMP Facility and a Translational Research Lab, and has extensive experience in the preclinical and clinical development and management of ATMPs, especially MSCs but also CAR T cells.

Trince, a spin-off from Ghent University, offers a unique intracellular delivery (transfection) technology for the life sciences/biotech field. The LumiPore platform is based on the interaction between pulsed laser light and photothermal nanomaterial. By irradiating the proprietary nanoparticles with laser light, highly localized light-induced thermal and mechanical forces are generated. When these forces come into contact with the cell membrane, they create temporary pores through which external effector molecules can enter the cell. This ‘photoporation’ technology was developed as a next-generation intracellular delivery platform for efficient, flexible gentle, and safe delivery of a wide variety of effector molecules in a broad range of primary and hard-to-transfect cells, while maintaining high therapeutic quality. The technology can deliver a diverse set of payloads in various hard-to-transfect cell types, including suspension and adherent cells (directly in a standard lab recipient) and even living tissue slices.

…
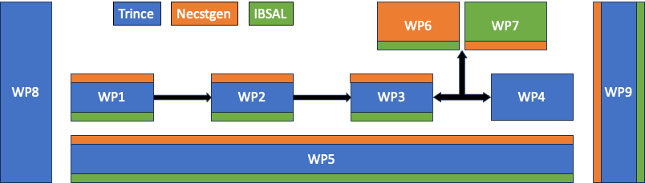
Developing the PEN photoporation platform entails 4 technical Work Packages (WP1-4). In WP1 the nanofibers are first optimized in a 96 well format for fast screening of different conditions. In WP2 the nanofibers will be incorporated in a microfluidics device for optimization of fluidic conditions to achieve a homogeneous distribution of cells on the nanofiber substrate and efficient collection of cells after transfection. Next, in WP3 the PEN-cartridge will be developed based on insights from WP1 and WP2, while in parallel the PEN-LumiPore device will be developed in WP4 for treatment of cells in the cartridge. WP5 covers aspects related to working towards cGMP compliance and runs in parallel with technology development. In WP6 and 7, the PEN photoporation platform will be externally validated in cGMP compliant infrastructures. The project is further supported by two pillars, being business development in WP8 and project management in WP9. All partners are collaborating closely during the entire project, carrying out tasks according to their respective expertise.
At the end of the project the partners aim to have a GMP ready automated high-throughput PEN photoporation platform with a throughput of >109 T cells and >107 MSCs per hour supported by a positive report on Leachables and Extractables for the disposable fluidic cartridge and a quality manual reviewed and approved by an external expert.
The Penphomet project represents a significant advancement in improving the accessibility and affordability of cell therapies, with the potential for far-reaching impacts on cancer treatment and beyond.
Ottergemsesteenweg Zuid 731 | 9000 Gent | Belgium
Privacy Policy | Terms & Conditions | Cookie policy